Introduction
In the race to develop a vaccine, the UK was the first country to approve the COVID-19 vaccine, less than a year after the UK’s first reported case. In a rare win during the pandemic, the UK are also current leaders in terms of total vaccination provision compared to other European countries. At the time of writing, three vaccines have been approved in the UK, more than any other country in the world, with more vaccines nearing the end of their trials, also showing promising efficacy data.
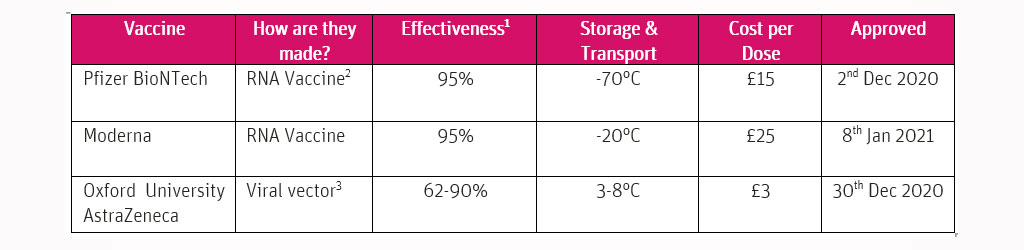
Table 1: Approved COVID-19 Vaccines In The UK
- [1] Based on trial data
- [2] RNA Vaccines use part of virus genetic code
- [3] Viral Vaccines use genetically modified virus
Lessons Learned
So can we learn from the pharmaceutical industry, particularly in the development of the COVID-19 vaccine? How did they invent, develop, test and deploy a vaccine in a tenth of the usual time? What contributed to this success and can we use any of their initiatives to speed up concept development within the Air Traffic Management (ATM) industry?
It is not the first time we have tried to learn from the pharmaceutical industry.
When the Air Traffic Management industry was trying to define what validation was and describe a validation process to aid concept development, we first researched what other industries did. The pharmaceutical industry had already done a lot of research about validation. They defined validated analytical methods to support drug characterisation and development. These ensured fitness for purpose and reliability of results. We used their principles to help create an ATM validation methodology which is now documented in the European Operational Concept Validation Methodology (E-OCVM). The E-OCVM, which was co-written by Think Research, was mandated to provide a common, structured and transparent understanding of what is required to perform validation to support concept development all the way through towards implementation.
Validation Definitions In Pharmaceutical & ATM Industry
“Process validation is defined as the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process.” – FDA
“Validation is an iterative process by which the fitness for purpose of a new system or operational concept being developed is established.” – E-OCVM
This demonstrates that we can learn from other industries and it can save us time and effort. Therefore, when we hear of a successful case study such as the COVID-19 vaccine development, it is important to know if any lessons can be brought over to research and development within ATM. Below are five lessons learnt and how they are relatable to us and the aviation industry in terms of concept development.
1. Promote Global Cooperation
Under normal circumstances, a vaccine typically takes up to 10-15 years due to the complexity of vaccine development. Vaccines work by training our immune system to remember an infectious agent without having to compromise the immune system or contract the infection. However, in March 2020, when the COVID-19 outbreak turned into a global pandemic, we needed a solution fast. Waiting 15 years would have caused an even more disastrous impact on society and also the economy. This spurred global cooperation for researchers to share their coronavirus data with other scientists which accelerated development.
This notion of global collaboration and sharing expertise and data is also important during concept development within the ATM industry. Recent collaborations and programmes that share research studies and lessons learnt are shown to fast track development and produce a solution that is more successful as they have had the insight from leading experts. One example of this is organisations such as CANSO who encourage members to work together around the globe to continuously improve Air Traffic performance.
In addition, programmes such as SESAR includes 100 organisations who work together with an aim of delivering next generation high performing technological and operational solutions for uptake by the aviation industry. Many SESAR solutions have now been implemented in local environments. For example, Extended Arrival Management (E-AMAN) is now deployed at London Heathrow in the form of Cross Border AMAN (XMAN) and is delivering improved traffic management. This solution is part of synchronised deployment plans (PCP) which have been implemented in 7 other locations in Europe. In addition, in 2014 the world’s first SESAR remote tower services (RTS) obtained operational approval in Sundsvall, serving Örnsköldsvik airport 150km away. Whilst this implementation took place outside of SESAR, it would arguable not have been achievable as quickly without the collaboration between multiple ANSPs and stakeholders from around Europe offering insight and expertise.
It is therefore clear that we should learn from the COVID vaccine and promote global collaboration where possible in order to share knowledge and experience to fasten development.
2. Build upon existing work
One of the biggest misconceptions of how “quickly” the vaccine was produced is regarding when the work on the vaccine actually started. SARS-COV-2 (COVID-19) is a member of the coronavirus family, of which there are hundreds– including four that cause the common cold. In addition, the coronavirus had already tried to jump from animals to humans twice in the past 20 years with the SARS epidemic in 2002 and MERS in 2012. Scientists have therefore been studying coronavirus for over 50 years – they had existing data on the structure, genome and life cycle of the virus so had a head-start. They knew how to work with the common features (the “spikes”) of a coronavirus.
The same principal applies in ATM concept development. Rarely do you have to start right from a blank piece of paper. Can you evaluate existing concepts/technologies and see how they can be improved or adapted rather than start from the beginning?
One recent and relevant example of this is Time Based Separation (TBS) which also evolved from previous ideas and research. This resulted in a TBS tool being developed at Heathrow in 2015 based on Time Based UK 6 category wake separations delivered to 4DME. This then evolved to enhanced Time Based Separation (eTBS) in 2018, taking into account RECAT-EU wake separations as well as support for Runway Occupancy Time. In addition, these changes were enabled by Optimised Runway Delivery that takes into account aircraft catching each other up as the lead aircraft slows down for landing. To further increase efficiency while maintaining safety, the existing concept is being expanded to Pairwise Separation (PWS) which will provide safe separation or spacing between aircraft depending on the specific aircraft types. TBS may have taken years to go from idea to implementation but PWS is aiming to do the same thing in a fraction of the time as it is building on pre-existing systems, tools and a wealth of experience.
Quite often the ideas are already out there. They may not have been successful first time around or the timing might not have been right; they might need to be “upcycled” or repurposed; or it might be that an adaptation of the design will make them applicable to entirely novel or wider use cases.
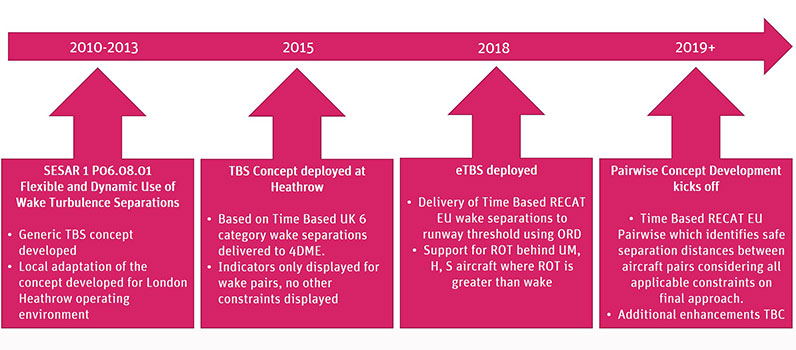
Figure 2: Concept Development Evolution of TBS at Heathrow
3. Encourage Innovation
Another lesson learnt is regarding innovation and not sticking to the existing method of development, by continuously thinking if there is a cheaper, better, or more efficient way of doing things.
In the past, vaccines have been developed by weakening or killing the virus or producing a part of it in the laboratory. This can be time consuming. The current COVID-19 vaccines have been developed using a new and revolutionary style of “plug and play” vaccine which is not only fast but also flexible. This involves slotting genetic material from the virus into a tried and tested delivery package. Once introduced into the human body, this generic material is used by the protein making machinery in our cells to develop the coronavirus spike protein which triggers an immune response.
This ‘plug and play’ style of development could also be adopted during concept development for future air traffic management systems. If controller tools and systems are adaptable in the future, then new functionality could be bolted on top. This would not only save money as the bulk of the system is pre-existing but it could also mean development time is accelerated. TopSky ATC developed by Thales is a good example of a modular system with interoperable components, covering a full range of functionality to suit the user and their needs.
Indeed, as the ATM infrastructure shifts to the distributed architecture proposed by SESAR’s Airspace Architecture Study we will need to see a shift from standardising hardware systems to standardising services implemented as software components operated on commercial hardware platforms. The infrastructure developed to support concept validation through Very Large Scale demonstrators can then also be used to support standards validation and interoperability testing prior to deployment using the ETSI Plugtest philosophy which has successfully accelerated standards development in areas such as mobile telecoms.
4. If you don’t have large investment, prioritise/balance resource
One of the most obvious reasons for the COVID-19 vaccine success was due to the huge amount of global investment. This meant that the vaccine development was prioritised with all resources and focus being dedicated to it. Normally investors would want to see evidence of any benefits before “passing go” and sinking more funding into the next stage of development. However, there was no time to wait and governments and private sectors were throwing millions into the hunt for a vaccine. Moreover, calculated risks were also taken. Vaccine manufacturing was carried out before the results from the clinical trials were received, in the hope it would deliver the anticipated results and would get the necessary approvals. The risk vs reward picture was fundamentally altered and what constituted “sure enough” was re-evaluated. Another important factor which should not be dismissed is the fact that multiple companies and vaccine platforms were being invested in at once, increasing the odds of a vaccine becoming available at the end of 2020.
The same level of urgency of development of an ATM concept would be welcome, although unexpected! However, it does demonstrate that if you have proper incentives, targeted investment and all necessary resource from validation staff to human performance experts to safety to engineers and operational staff to name a few, moon shots are achievable. In our industry, many projects get delayed due to the fact that other projects are also competing for resource impacting limited resource and infrastructure such as Real Time Simulation (RTS) availability. Historically, air traffic controller availability has also been difficult, as the vast majority perform an operational role and have to be taken off the rota to support concept development activities such as workshops or RTS.
With the current investment cycles in ATM modernisation it is unlikely that we will be able to take such a large yet agile approach, but more efficient use of current resources and prioritisation depending on the urgency of the project is key. With the decline in air traffic due to COVID 19, now is the perfect time to use all the available resource of staff that are on furlough and instead use them into helping develop concepts – if the ANSP budget allows.
5. Engage stakeholders early
Although we have already discussed calculated risks being taken which resulted in processes being sped up with the vaccine development, there were also other overlapping stages in the validation process. Typically there are three different phases of human trials in vaccine development, each growing in number and broadness of willing volunteers. In addition, with each phase, more data is gathered to provide safety evidence such as possible side effects, effectiveness, dosage levels and information regarding how the disease could be prevented in the future.
During the development of the COVID-19 vaccine, many of these trials were run almost back-to-back. This is novel as it normally takes years between trials due to bureaucracy trying to get approvals and negotiating with manufacturers and trying to get enough people to participate. In addition, the approval process is expensive and risky so typically vaccination developers want to wait to make an application until all their ducks are in a row. However, regulators were engaged throughout. They were conducting “rolling reviews” of safety, manufacturing standards and effectiveness throughout as information was released to regulators as soon as it was gathered.
Within the E-OCVM, we always air caution into rushing through or overlapping stages in development. It can be risky and potentially not worth it unless you have extremely high confidence in the concept and/or it has been done elsewhere and you are adapting it for a specific environment. But some of the initial stages of development may be fast-tracked if the basic concept has already been proven to work. This way, focus can be gathered towards implementation. Moreover, the pharmaceutical industry has shown us that stakeholder engagement throughout the entire concept development lifecycle is critical. Both industries involve development of concepts which are safety critical so safety approval cannot be short cut. However, by engaging safety experts, human performance and validation experts from the get-go ensures they are part of the validation process from the start and ensures the evidence gathered during any validation exercises satisfies their needs which will lead to a higher chance of success.
Conclusion
Overall, I believe we can learn many lessons from the pharmaceutical industry when it comes to speeding up ATM concept development. It is typical for our concepts to take just as long as vaccines used to take before the global pandemic erupted and changed our world. There may not be the same drive for a new ATM system as there is for a vaccine in a pandemic so there will never be the same sense of urgency. However, there are some aspects we can learn from such as making more efficient use of resources if we do not have the money to throw into development, building upon existing concepts, fast tracking certain areas of concept development and ensuring stakeholders are engaged throughout.
Much like a vaccine, innovation within Think’s bloodstream (!!!) and we are always looking for quicker, better and cheaper ways of doing things. If you would like us to help you with concept development or need help with validation, please get in touch.

Author: Sarah McLarty, Senior ATM Consultant


Recent Comments